Density: (SI symbol ρ, greek letter "rho")
![]()
m = mass usually in grams
V = volume usually in mL
Percent: Percent in chemistry is mass percent unless stated otherwise.
For percent of substance "A":
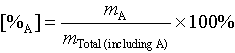
Relationship between number of things, N, and number of moles, n.
N = NA n where: NA = 6.022 x 1023 mol-1
Relationship between moles and mass
![]()
where
M is the molar mass (from the periodic chart) with units of g mol-1
m is the mass in g
n is the number of moles
Stoichiometric relationship in a reaction:
aA + bB + ... --> cC + dC
![]()
i.e. the number of moles reacting are proportional to the reaction stoichiometric numbers.
See also the mole map
Definition of molarity, C.
C of a solute is related to the number of moles of the solute, n, and the volume of the solution by:
![]()
where V is in units of L.
Use of parametric equations:
Example, dilution of a solution.
A certain volume of a solution is initially at V1 . The concentration of this solution is C1 . The solution is then diluted by the addition of more solvent until the volume is V2. To calculate the concentration C2 one uses the parametric approach.
In this problem, n remains constant since no solute is either added or removed. Thus the before and after equations for molarity may be written:
![]()
For both equations, place all the variables on one side of the equation (here on the left) and all that remain constant on the other side (here on the right) so:
C1V1 = n and C2V2 = n
Since the right sides are the same, then the left sides are equal:
C1V1 = C2V2
This equation may be used to calculate dilutions of solutions.
(Note: the final volume is very often approximated by summing the initial volume and the volume of the solvent added. This is often very close to correct, especially for solutions which are dilute - say 1 M or less.)
Titration formula for a titration where the acid, subscript A, and base, subscript B, have reaction stoichiometry coefficients of nA and nB.

Caution, this looks like the above dilution formula but with nA and nB it is not the same!
PV = nRT where R = 0.08206 L atm mol-1 K-1
Parametric use of the Perfect Gas Equation leads to several other laws.
Example - Boyle's law:
P1V1 = P2V2
Example - Charles' law: 
Example - Avagadro's law: 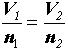 and others.
and others.
Combining the Perfect Gas Equation with reaction stoichiometry.
Use the parametric trick to solve stoichiometry problems. For example, Avogadro's law as applied to a reaction (constant P and T )
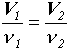
or a similar law when the volume and temperature are held constant.
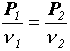
Dalton's law:
since: ntotal = nA + nB + nC + . . .
Then the ideal gas equation would predict that
Ptotal = PA + PB + PC + . . .
Graham's law relates the velocity of effusing or diffusing gases, v , to their molar mass, M, by:
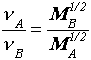
Reaction stoichiometry used with the Ideal Gas Law:

Henry's Law: PA = KH CA
Definitions of concentration units:
| molarity: | CA = nA/Vsolution V is in L |
| molality: | bA = nA/m solvent m is in kg |
| mole fraction: | XA = nA/ntotal |
| percent | [%A] = 100% * mA/m total |
conversion tables for concentration units
Colligative Properties:
| Freezing Point Depression: | ΔT = Kfbtotal |
| Boiling Point Elevation: | ΔT = Kbbtotal |
| Raoult's Law: | PA = XAPo A A = solvent |
| Osmotic Pressure: | Π = CsoluteRT |