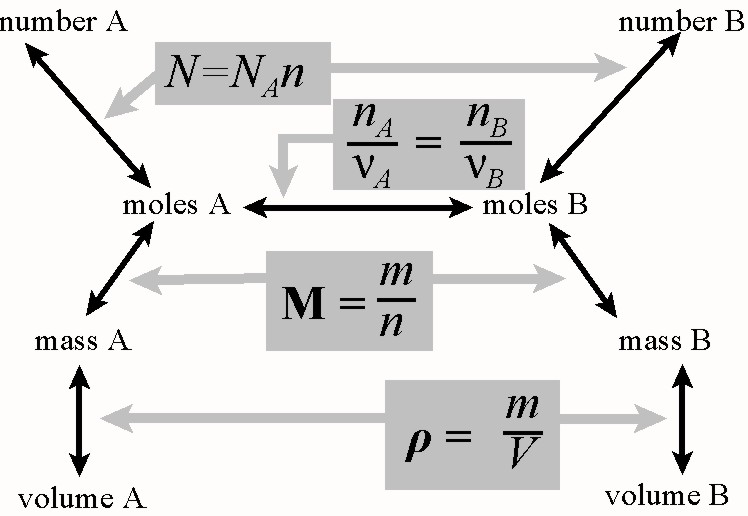
Units or values for the above physical quantities are as follows:
N: is number of things, i.e. atoms, moleculesor particles
NA = 6.022 x 1023 mol-1
n is number of moles. Unit: mol
m is mass. Unit: g
M is the molar mass (molecular mass, atomic mass or formulamass - older designations: molecular weight, atomic weight.) Unitsg mol-1
ρ is the density usually with unitsof g mL-1 or equilantly kg L-1
ν is the reaction stoichiometric number(coefficients used in the balanced reaction) and is an exact number.