Methane
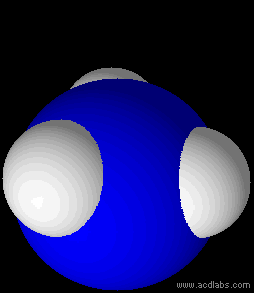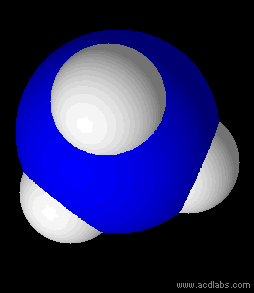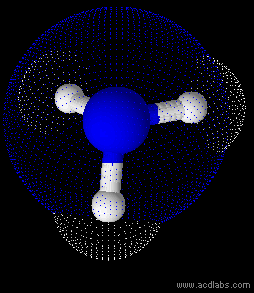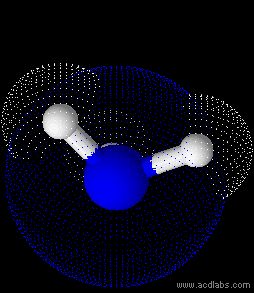
Ammonia
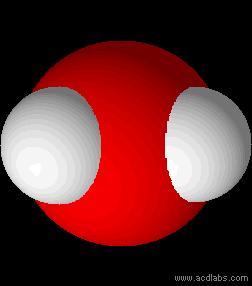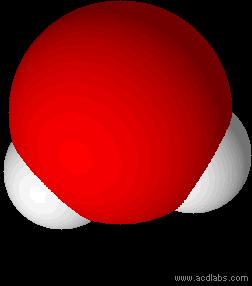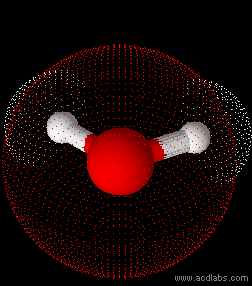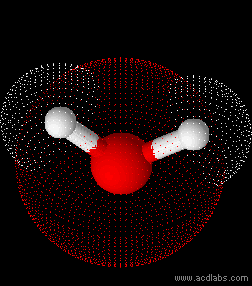
Water
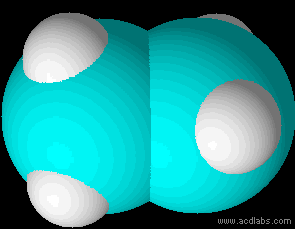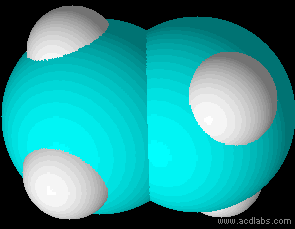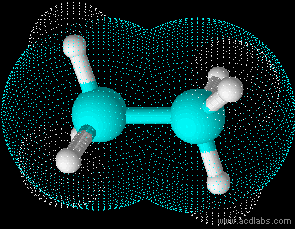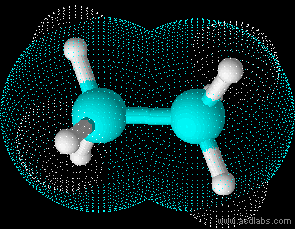
Ethane
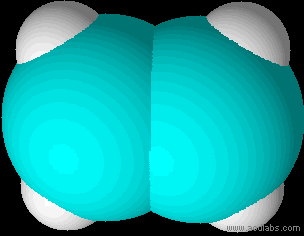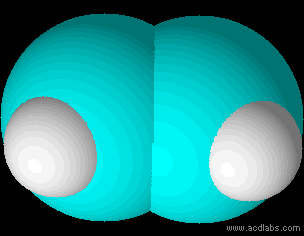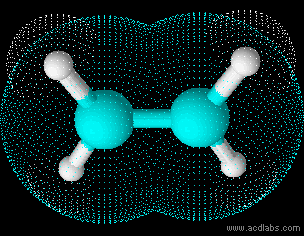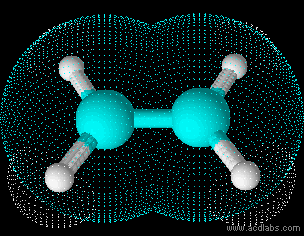
Ethene
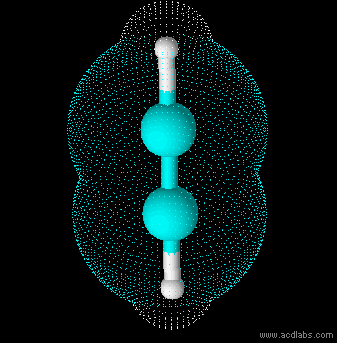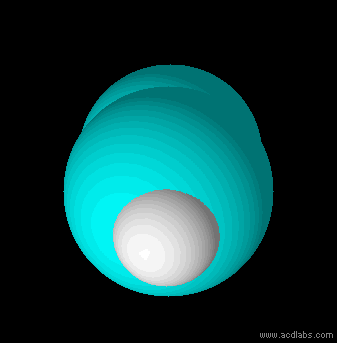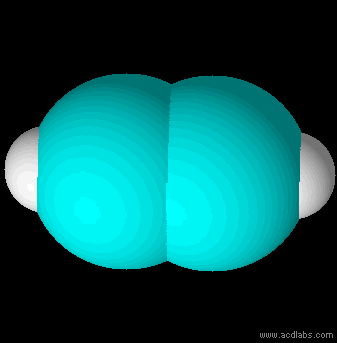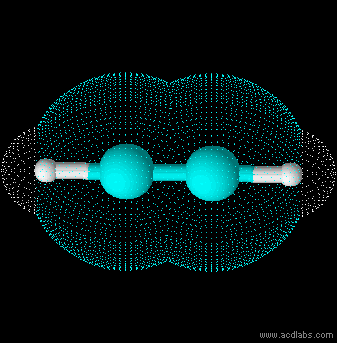
Ethyne
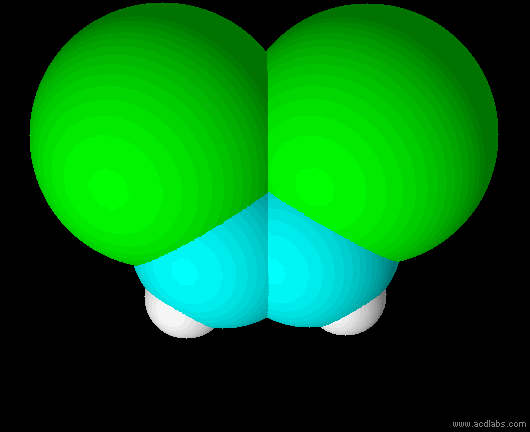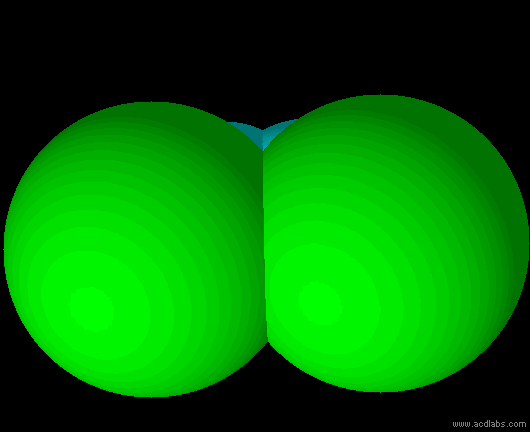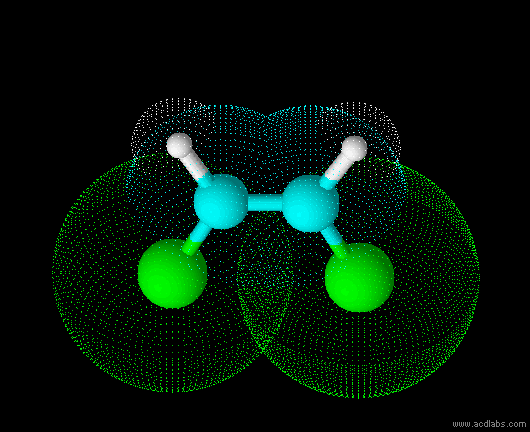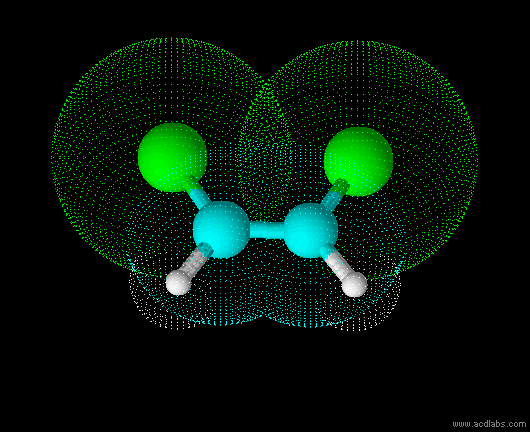
cis-dichloroethene
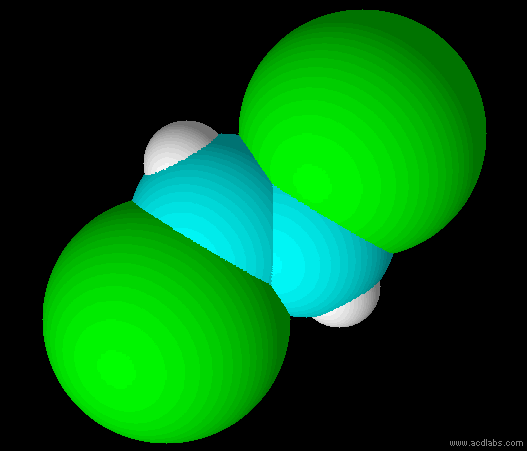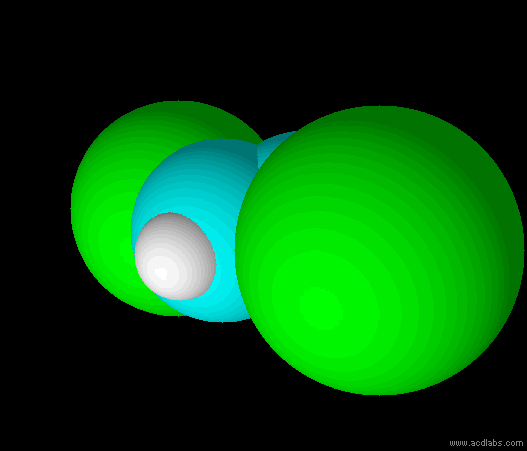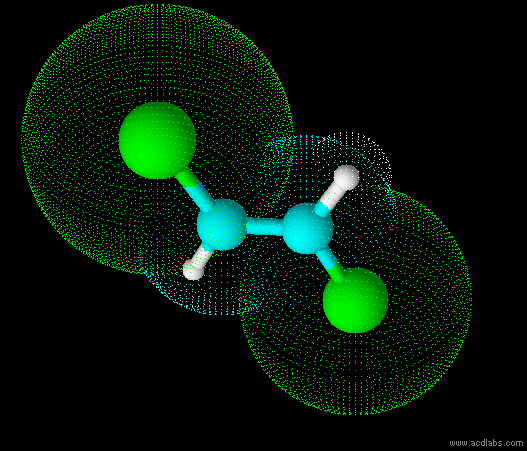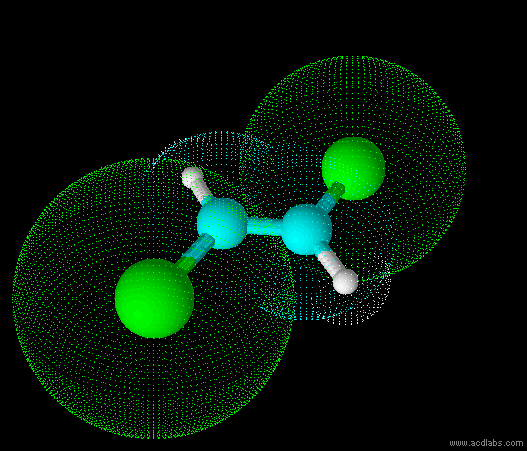
trans-dichloroethene
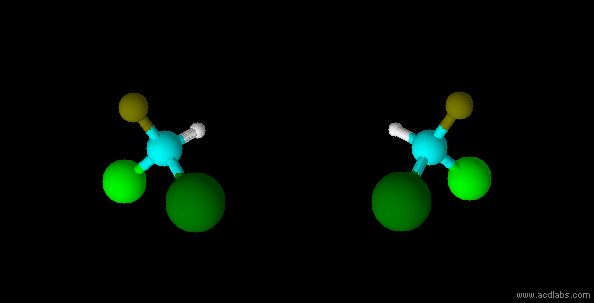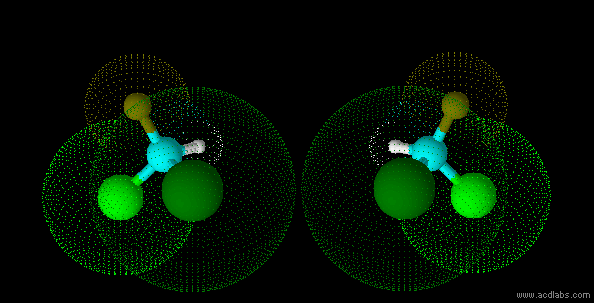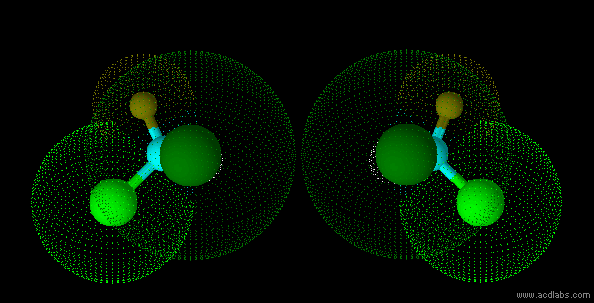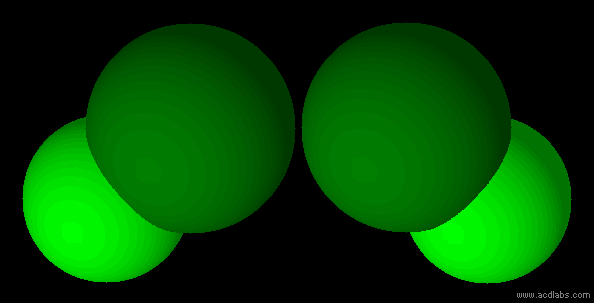
stereo isomers
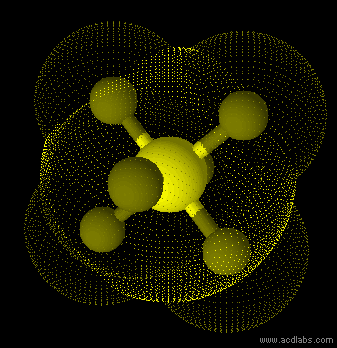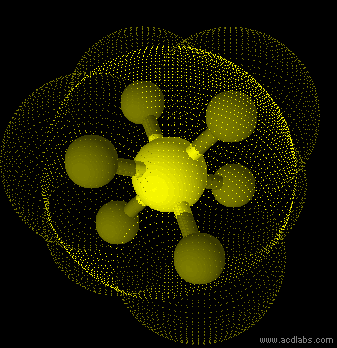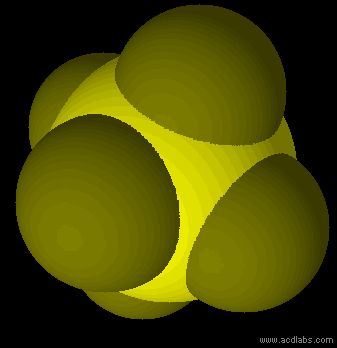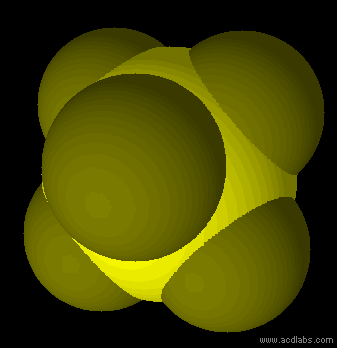
SF6 - sp3d2 - octahedron - non-polar
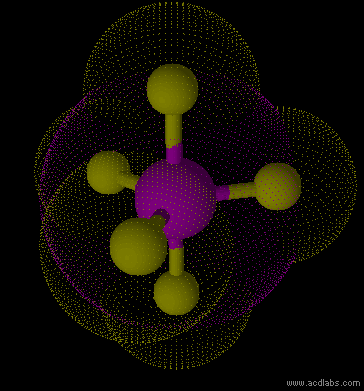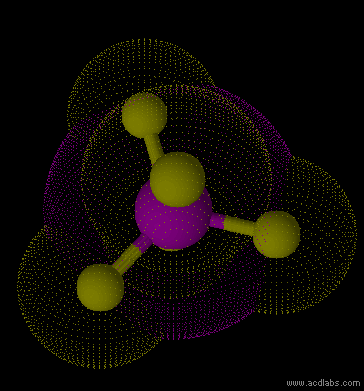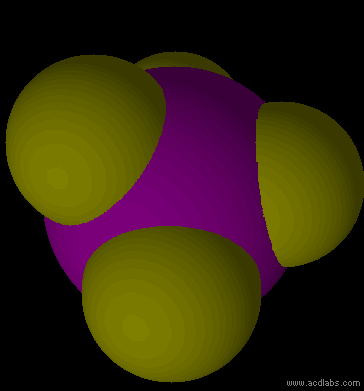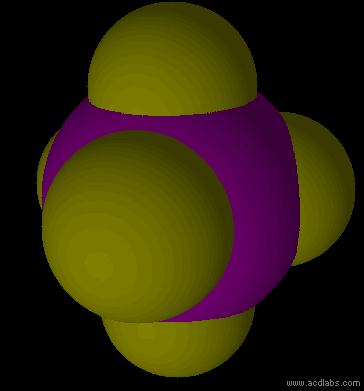
PF5 - sp3d - trigonal bipyramid - non-polar
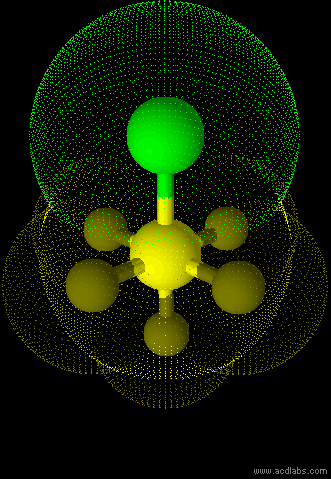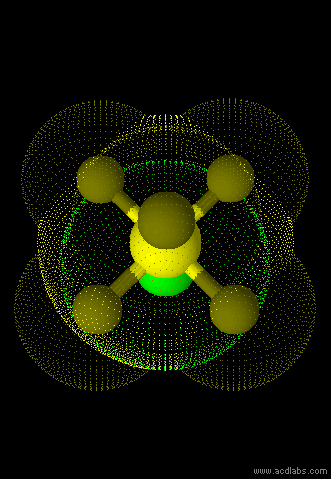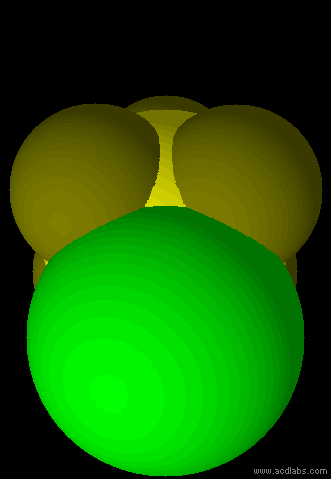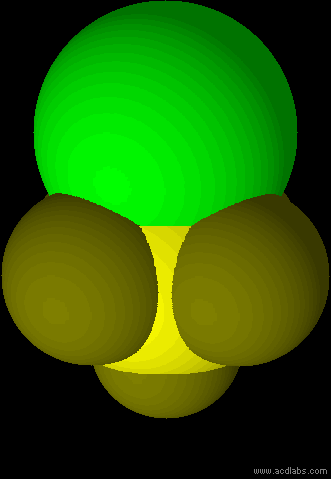
SF5Cl - sp3d2 - octahedral - polar
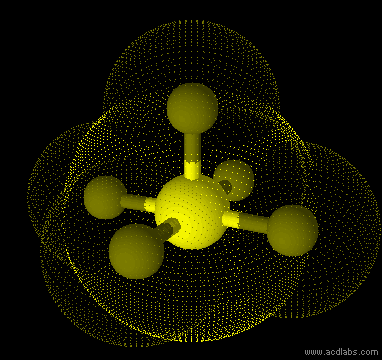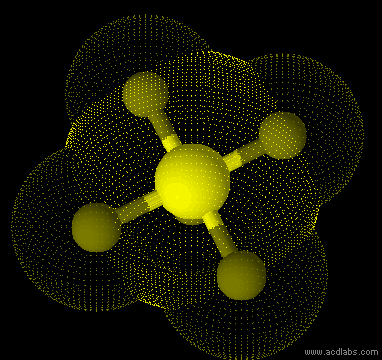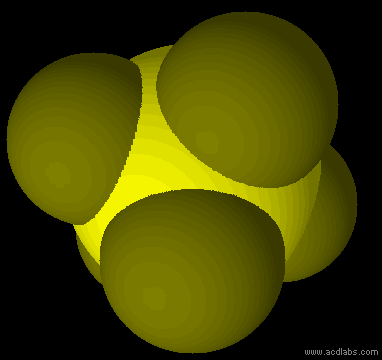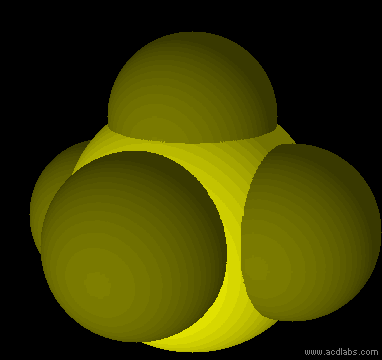
SF5- - sp3d2 - square pyramid - polar
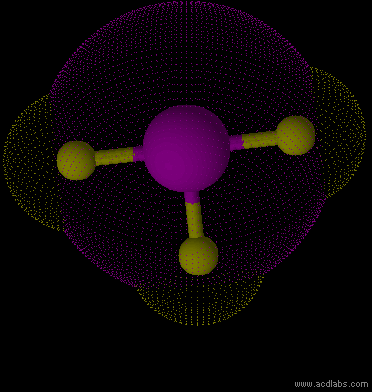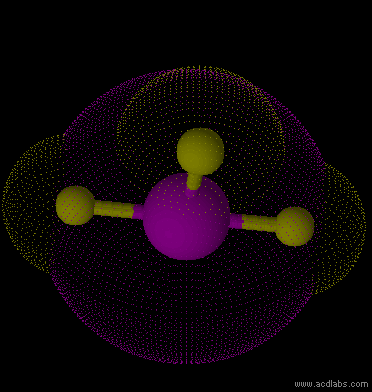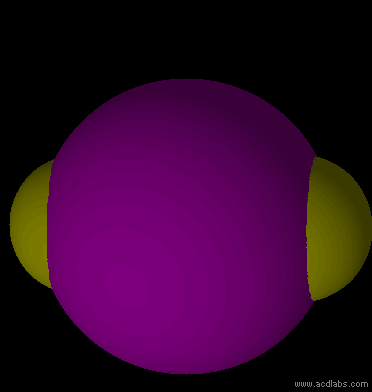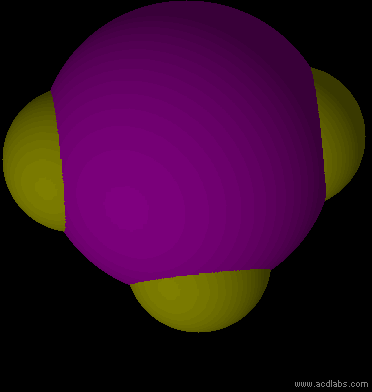
IF3 - sp3d - T-shaped - polar
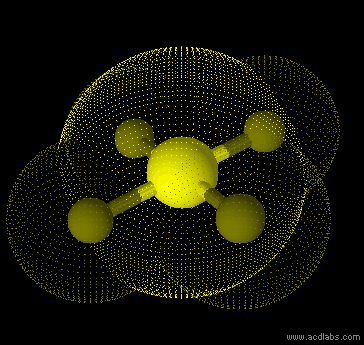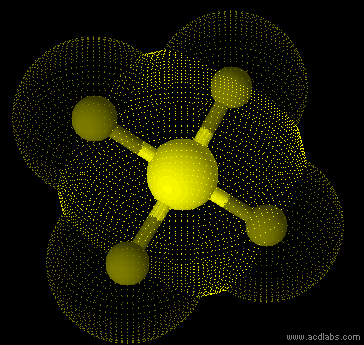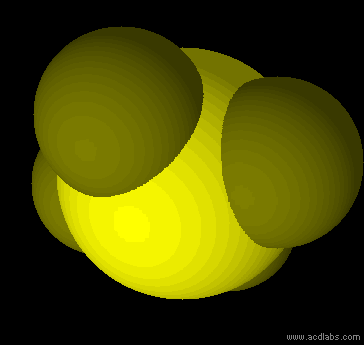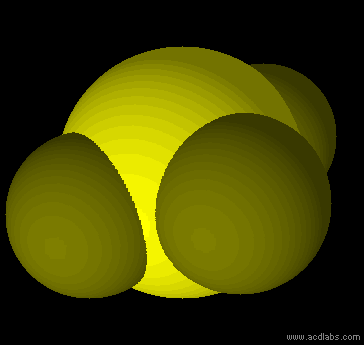
SF42- - sp3d2 - square planar - non-polar