Calculating Dilution Problems
The most common concentration unit used in chemistry is molarity. The
following equation defines molarity.
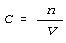
In this equation, n is the number of moles of the species dissolved
in the solution. This dissolved material is called the solute. V
is the volume of the solution - the total volume with the solute in it.
C in this equation stands for molarity. The unit symbol for this
is m.
In a dilution, one starts with a solution of one concentration and adds
solvent. (For example, one would add water.) One then obtains another solution
that is less concentrated. In this process, one losses none of the material
dissolved (solute.) Only the volume and concentration changes.
Example:
Vinegar is a 0.80 m solution of acetic acid. What is the concentration
of acetic acid if one mixes 50 mL of vinegar with 450 mL of water? From
this one gets 500 mL of solution? (Note that the final liquid volume is
the sum of the two solutions together for dilute water solutions.)
In the above example, the concentration of acetic acid goes down due
to dilution. How would one calculate the number for molarity?
This is a typical problem that requires use of parametric equations.
To use these, one must discern what is remaining constant and what is changing.
The volume and concentration change.
The number of moles solute remain constant.
Rearrange the above equation as follows. Place all variables which remain
constant are on one side of the equal sign. Place all variables which change
are on the other side. This gives the following.
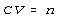
Do this for the starting condition, subscripted s, and for the final
condition, subscripted f.
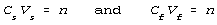
Equate the two conditions by eliminating n between the two.
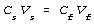
Substitute, being careful to use the correct final volume. For the above
example, this yields the following.
(0.80 m)(50 mL) = Cf(500 mL)
Thus: Cf = 0.080 m